Premarket 510(K)/PMA Approval
Accelerate Premarket Cybersecurity Approval
Built on a decade of expertise and regulatory submissions, ELTON is an FDA-recognized firm. Backed by an
FDA-approved Medical Device Development Tool (MDDT)
ELTON AI
Meet Global Cybersecurity Requirements
ELTON automates compliance with 2026 FDA Premarket and 2025 AI-Enabled Device Cybersecurity Guidance, delivering a seamless transition from submission to continuous postmarket security. ELTON is backed by an FDA-Approved Medical Device Development Tool (MDDT), ensuring error-free regulatory review.
Avoid Late-Stage Delays
ELTON integrates vulnerability analysis early in development, enabling each software release to improve security posture, meet FDA expectations for SPDF, and reduce risk well before submission, minimizing late-stage rework and accelerating regulatory approval.
Premarket Approval with Postmarket Readiness
When ELTON is engaged during premarket development, it builds a deep understanding of your product’s architecture, software components, and risk posture well before submission. FDA requirements mandate a Postmarket Monitoring SOP at submission, ELTON ensures monitoring begins early and continues seamlessly, eliminating audit gaps.
Contact Us
ELTON AI Identifies Vulnerabilities That Matter
Support vulnerability decisions with a traceable, FDA-approved solution.

Why ELTON
1000+ Products Approved with ELTON
We’ve assisted thousands of products and interfaced with global regulators.
As the leading Medical Device Penetration Testing Firm, regulatory success starts at premarket. ELTON enables defensible approvals through secure design decisions, obtaining clearance while reducing long-term postmarket risk, avoiding expensive recalls, by design.
Regulations
ELTON Meets Global Cybersecurity Regulations
Do it right the first time, for all markets
Starting in 2014 the US FDA released premarket guidance for cybersecurity and further introduced an update in 2023 and 2025 with expansive additions. The US FDA released postmarket cybersecurity guidance in 2016 that remains in effect today.
In the EU, 74/2017 (MDR) and 746/2017 (IVDR) called the Medical Device Regulations are requiring all medical devices sold in the EU be recertified to enhanced cybersecurity standards. The Medical Device Coordinating Group (MDCG) provided guidance for meeting the EU Medical Device Regulation (MDR) as it pertains to cybersecurity (MDR MDCG 2019-16).
China (CFDA) Cybersecurity Law (CSL) is the administration of medical devices in China, where as of 2018 medical devices must be assessed for cybersecurity protection under the Principles on Guiding Technology Examination of Medical Device Cybersecurity Registration (CFDA Guidelines).
Japanese regulation stipulates that, in addition to the conformity to the JIS T 2304 (IEC 62304), for medical devices connected to other IT devices and medical devices connected to the Internet, cyber security measures based on JIS T 81001-5-1 (IEC 81001-5-1) are required to reduce cyber security risks to acceptable levels. This new regulation was put into practice on April 1, 2023, with a one-year transitional period until March 31, 2024.
No More Spreadsheets
Conflicting documentation will result in outright rejection and a lifecycle of vulnerabilities.
Vulnerabilities are often fragmented across tools, teams, and spreadsheets, creating inconsistent documentation and regulatory risk.
ELTON serves as the single pane of glass for all, unifying theoretical threat modeling with true vulnerability analysis, as a single source of all vulnerability discovery and management.
Software Bill of Materials Testing
Source Code Testing
Known Vulnerability Scanning
Penetration Testing
Fuzz Testing
Robustness Testing
Resiliency Testing
Threat Mitigation Testing
Meet FDA Guidance for AI-Enabled Devices
The FDA’s January 2025 draft guidance on AI-Enabled Device Software Functions (Artificial Intelligence-Enabled Device Software Functions: Lifecycle Management and Marketing Submission Recommendations) includes cybersecurity requirement that should be keeping medical device startup teams up at night. The FDA has made it explicit what many NextGen AI-Enabled devices have been hoping they could avoid: cybersecurity testing for AI-specific vulnerabilities that exist well beyond the trained model at runtime.
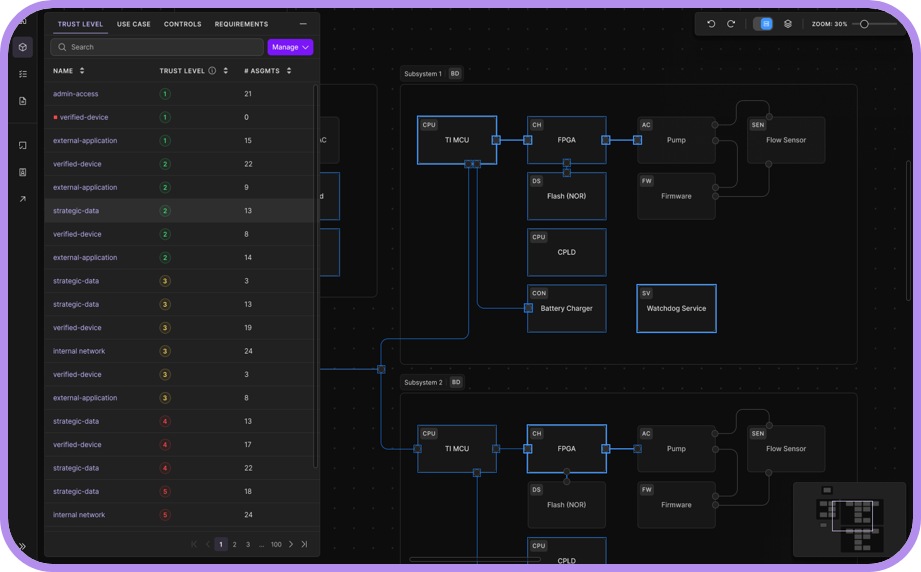
Intelligence
Where Industry Expertise Meets AI Automation
ELTON is powered by insights from over a decade of medical device testing expertise and 1000+ FDA-approved submissions. View FDA Cybersecurity Guidance
Streamlined Premarket Approvals Start with ELTON
Design, develop, and ship medical devices faster by employing ELTON, backed by an FDA-approved Medical Device Development Tool (MDDT)
